CMS Proposed Rule Aims to Close ESRD Health Equity Gaps

Summary
CMS is making its first direct effort to decrease health equity disparities and improve rates of home dialysis and kidney transplant among socioeconomically disadvantaged patients.
The Centers for Medicare & Medicaid Services (CMS) has taken a welcome proactive stance in addressing significant, persistent and costly inequities in U.S. health outcomes. As part of this effort, for the first time the agency is directly addressing health and socioeconomic disparities as major contributors to chronic kidney disease and end-stage renal disease (ESRD), and the cost and quality of care that these patients receive. On July 1, CMS issued a proposed rule to update the ESRD Prospective Payment System (PPS) for calendar year (CY) 2022, that has important implications for dialysis technology innovators, providers and patients.
Among its many provisions, the rule proposes changes to the ESRD Treatment Choices (ETC) Model, the mandatory payment model that is focused on encouraging greater use of home dialysis and kidney transplants, to urge dialysis providers to decrease disparities in rates of both options among patients from minority and low-income populations.
This proposed rule is an encouraging tailwind for the movement to home dialysis, as momentum and clinical evidence builds to support a larger percentage of ESRD patients being educated about this treatment option that helps them realize benefits such as greater autonomy and better quality of life. As noted in a recent NEJM Catalyst commentary co-authored by Outset Medical’s Head of Government Affairs, Tonya Saffer, for complex reasons, most dialysis is delivered in-center even when home dialysis is feasible and preferable, and people of color and lower-income populations are well documented to be less likely to initiate dialysis with a home therapy.
A CMS First: ETC Model Modifications Directly Address Health Equity
CMS’ rule proposes several first-of-their-kind modifications to the mandatory ETC Model, that began in its original form this past January. The changes are designed to directly address and help close health equity gaps and encourage home dialysis as an option for ESRD patients of lower socioeconomic status. Proposed changes to the ETC model build on the current model by proposing to test a new approach that rewards ESRD facilities and Managing Clinicians for achieving significant improvement in the rates of home dialysis as well as kidney transplants. The rule includes a 10% increase to the home dialysis and transplant benchmark rates every two measurement years and a health equity incentive for increasing these rates among dual-eligible (Medicare and Medicaid) or low-income recipient beneficiaries.
CMS is considering a two-tiered approach to address disparities in home dialysis and transplant rates through the ETC Model’s benchmarking and scoring methodology:
- The agency is proposing to add a Health Equity Incentive to the improvement scoring methodology for both the home dialysis and transplant rate. Through this Incentive, ETC participants who demonstrate significant improvement in rates of home dialysis or transplantation among their beneficiaries who are dual-eligible for Medicare and Medicaid or low-income-subsidy (LIS) recipients could earn additional improvement points.
- CMS is also proposing to stratify achievement benchmarks by proportion of beneficiaries who are dual-eligible or are LIS recipients, so ETC participants who see a high volume of these patients would not face negative financial consequences as a result.
Encouragingly in terms of its patient focus, CMS is also issuing a Request for Information (RFI) seeking information from the public about a beneficiary experience measure for home dialysis care. This includes:
- The In-Center Hemodialysis (ICH) Consumer Assessment of Healthcare Providers and Systems Survey (CAHPS)® survey that includes perceptions of changes in quality of care to beneficiaries who receive in-center dialysis.
- CMS is considering and seeking comments on an existing or new home dialysis measure that addresses patient satisfaction, patient activation and quality of life.
In another example of the agency’s focus on overcoming patient barriers, it is also proposing to waive certain requirements such that the Kidney Disease Education (KDE) benefit under the ETC Model can be furnished via telehealth, regardless of the location of the beneficiary or qualified staff.
TPNIES for CY 2022: Establishing Substantial Clinical Improvement
Also as part of the proposed rule, Outset Medical’s Tablo Hemodialysis System is one of two products, along with a peritoneal dialysis monitoring system from CloudCath, that are under consideration for the Transitional Add-on Payment Adjustment for New and Innovative Equipment and Supplies (TPNIES) for CY 2022. If approved, TPNIES would establish additional important payment mechanisms to help offset the cost to providers of investing in new technology. Specifically, for two calendar years for those home dialysis machines that receive TPNIES, TPNIES equals 65% of the price established by the Medicare Administrative Contractors (MACs), using the information from the invoice and other specified sources of information minus a $9.41 off-set to account for the portion of the bundle that already is allocated to pay for capital equipment.
The agency is interested in gaining feedback from stakeholders if there is agreement that the areas that Outset identified regarding its Tablo System represent substantial clinical improvements (SCI) relative to the current standard of care for home hemodialysis, identified as the incumbent NxStage home dialysis machine. Along with providing detailed technological and clinical evidence data, Outset stated in its application that the unique features of Tablo combine to provide a significantly differentiated hemodialysis solution with benefits designed to “address many system-related barriers that result in patients resigning themselves to in-center care and/or stopping home modalities due to the burden of self-managed therapy.” Outset also stated that the totality of evidence submitted in support of Tablo—as demonstrated by the company’s IDE trial—demonstrates SCI over the current standard of home dialysis care. This is also supported by Outset’s patient preference data, where 86% of prior NxStage patients in the Tablo IDE study found Tablo easier to use than the incumbent device and preferred to remain on Tablo at the end of the study (also see Figure 1).
Figure 1: Patient Device Preference for Home Hemodialysis: A Subset Analysis of the Tablo Home IDE Trial
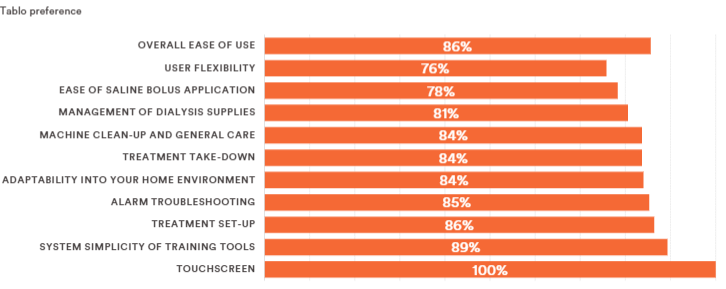
CMS responded in the proposed rule that “We understand that greater flexibility for patients in the way that they receive their dialysis treatments may represent a benefit to Medicare beneficiaries who are candidates to receive this treatment in the home setting. We invite comments on whether this potential benefit represents SCI, including whether the Tablo System represents an advance that substantially improves, relative to renal dialysis services previously available, the treatment of Medicare beneficiaries.”
If finalized, the changes in the proposed rule would take effect January 1, 2022. CMS is requesting public comments through August 31.
Additional Resources
Executive Order 13985 on Advancing Racial Equity and Support for Underserved Communities through the Federal Government
CMS ESRD PPS CY 2022 Proposed Rule (CMS-1749-P) Fact Sheet
Proposed Rule posted on the Federal Register
PDF Version of the Proposed Rule
Public Comments page on Regulations.gov

